Reliable diagnostic tools depend on credible reference materials
Design your respiratory diagnostic assay
Our extensive collection contains a variety of viral, bacterial, and fungal species for respiratory molecular diagnostics development, including high-priority organisms likely present in respiratory specimens. To support your unique assay development needs, we provide these materials in a variety of formats.
- Heat-inactivated preparations – Inviable, non-replicative preparations that are quantitated by Droplet Digital PCR or qPCR. These materials are ideal for use in molecular assays or as a process control (e.g., nucleic acid extraction through the qPCR amplification process).
- Live strains – Minimally passaged, fully characterized strains that are authenticated using a polyphasic approach that confirms genotype and phenotype.
- High-titer viruses – Purified preparations provided at an infectious titer of >107 TCID50/mL, CEID50/mL, or PFU/mL.
- Genomic nucleic acids – Whole-genome preparations aseptically prepared from minimally passaged ATCC cultures. Each preparation is supported by stringent quality control testing to ensure product authenticity and functionality.
- Synthetic nucleic acids – Quantitative DNA and RNA synthetically manufactured under ISO 13485 guidance to include key target regions from select viral, bacterial, and protozoan species. These standards enable researchers to work with higher concentrations of target nucleic acid sequences for unculturable or high-containment species.
- Respiratory inclusivity and exclusivity panels – ATCC’s respiratory inclusivity and exclusivity panels are composed of authenticated nucleic acids or heat-inactivated material derived from respiratory pathogens commonly cited for use as control materials in SARS-CoV-2 EUA submissions and/or FDA 510(k) submissions.
Explore our resources for respiratory molecular diagnostics development by product type
Table 1. Viruses
| Organism | Heat-inactivated Strains | Live Strains | Genomic Nucleic Acids | Synthetic Nucleic Acids |
|---|---|---|---|---|
| Adenovirus 1 | ✓ | ✓ | ||
| Adenovirus 4 | ✓ | ✓ | ||
| Adenovirus 7 | ✓ | ✓ | ||
| Bocavirus | ✓ | |||
| Cytomegalovirus (HHV-5) | ✓ | ✓ | ||
| Enterovirus A (EV-A71) | ✓ | |||
| Enterovirus B (Echovirus 6) | ✓ | |||
| Enterovirus C (Coxsackievirus A17) | ✓ | |||
| Enterovirus D (68) | ✓ | ✓ | ||
| Epstein-Barr virus (HHV-4) | ✓ | ✓ | ||
| Human coronavirus 229E | ✓ | ✓ | ||
| Human coronavirus HKU1 | ✓ | |||
| Human coronavirus NL63 | ✓ | |||
| Human coronavirus OC43 | ✓ | ✓ | ||
| Human herpesvirus 1 | ✓ | ✓ | ||
| Human herpesvirus 2 | ✓ | ✓ | ||
| Human metapneumovirus | ✓ | |||
| Influenza A virus subtype H1 | ✓ | ✓ | ||
| Influenza A virus subtype H3 | ✓ | ✓ | ||
| Influenza B virus | ✓ | ✓ | ||
| Measles virus | ✓ | ✓ | ||
| MERS-CoV | ✓ | |||
| Mumps virus | ✓ | ✓ | ||
| Parainfluenza virus 1 | ✓ | ✓ | ||
| Parainfluenza virus 2 | ✓ | ✓ | ||
| Parainfluenza virus 3 | ✓ | ✓ | ||
| Parainfluenza virus 4 | ✓ | ✓ | ||
| Respiratory syncytial virus | ✓ | ✓ | ||
| Rhinovirus | ✓ | ✓ | ||
| SARS-CoV | ✓ | |||
| SARS-CoV-2 | ✓ | ✓ | ✓ |
Table 2. Bacteria
Table 3. Fungi
| Organism | Heat-inactivated Strains | Live Strains | Genomic Nucleic Acids | Synthetic Nucleic Acids |
|---|---|---|---|---|
| Aspergillus flavus | ✓ | ✓ | ||
| Aspergillus fumigatus |
✓ | ✓ | ||
| Candida albicans | ✓ | ✓ | ||
| Cryptococcus neoformans |
✓ | ✓ | ||
| Pneumocystis jirovecii |
✓ |
Evaluate assay performance
Because molecular diagnostic assays provide powerful tools for the rapid detection and identification of clinically relevant pathogens, it is essential that they are properly validated to guarantee accurate results and uncompromised data. To evaluate the performance of a novel respiratory molecular diagnostic test, we provide NGS standards to help optimize your workflow as well as a variety of materials for validation studies on the limit of detection, inclusivity, and cross-reactivity of the assay. These studies help ensure that the assay is able to accurately detect your target without reacting to related pathogens.
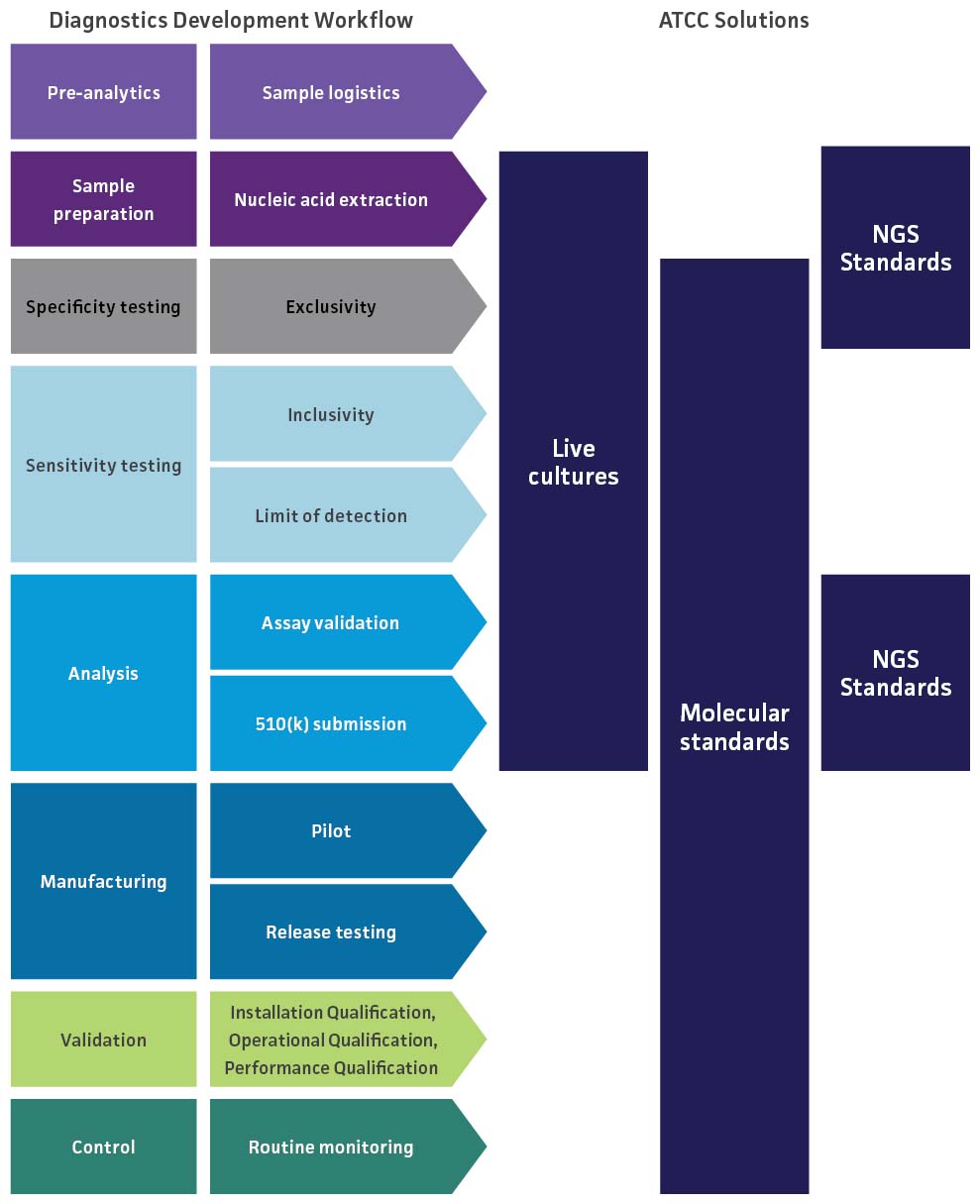
SARS-CoV-2 Molecular Diagnostics Development
The highly pathogenic nature and transmission dynamics of SARS-CoV-2 have necessitated the availability of rapid, robust detection methods to ensure that infected individuals are treated in a timely manner. For SARS-CoV-2 molecular diagnostics manufacturers moving from emergency use authorization (EUA) to 510(k) premarket submission to the US Food & Drug Administration (FDA), robust testing must be performed to demonstrate that the device is safe and effective as well as substantially equivalent to legally marketed devices. To support these validation studies, ATCC provides an extensive array of authenticated and clinically relevant materials for evaluating limit of detection, inclusivity, and cross-reactivity.
Develop your SARS-CoV-2 assay